Here are the comprehensive Chemical Reactions and Equations Notes, based primarily on the NCERT textbook for the CBSE Class 10 Science syllabus, Chapter 1. These notes cover all key concepts of the chapter, including definitions, types of chemical reactions, chemical equations, balancing methods, and important real-life examples. Designed to help you grasp each topic clearly, the notes include simple explanations, activity-based examples, and relevant diagrams (where applicable). Whether you are revising for exams or building a strong foundation, this chapter summary is your quick and reliable guide.
1.0 Introduction to Chemical Reactions

- Definition: A chemical reaction is said to have taken place whenever a chemical change occurs. Chemical reactions involve the breaking and making of bonds between atoms to produce new substances.
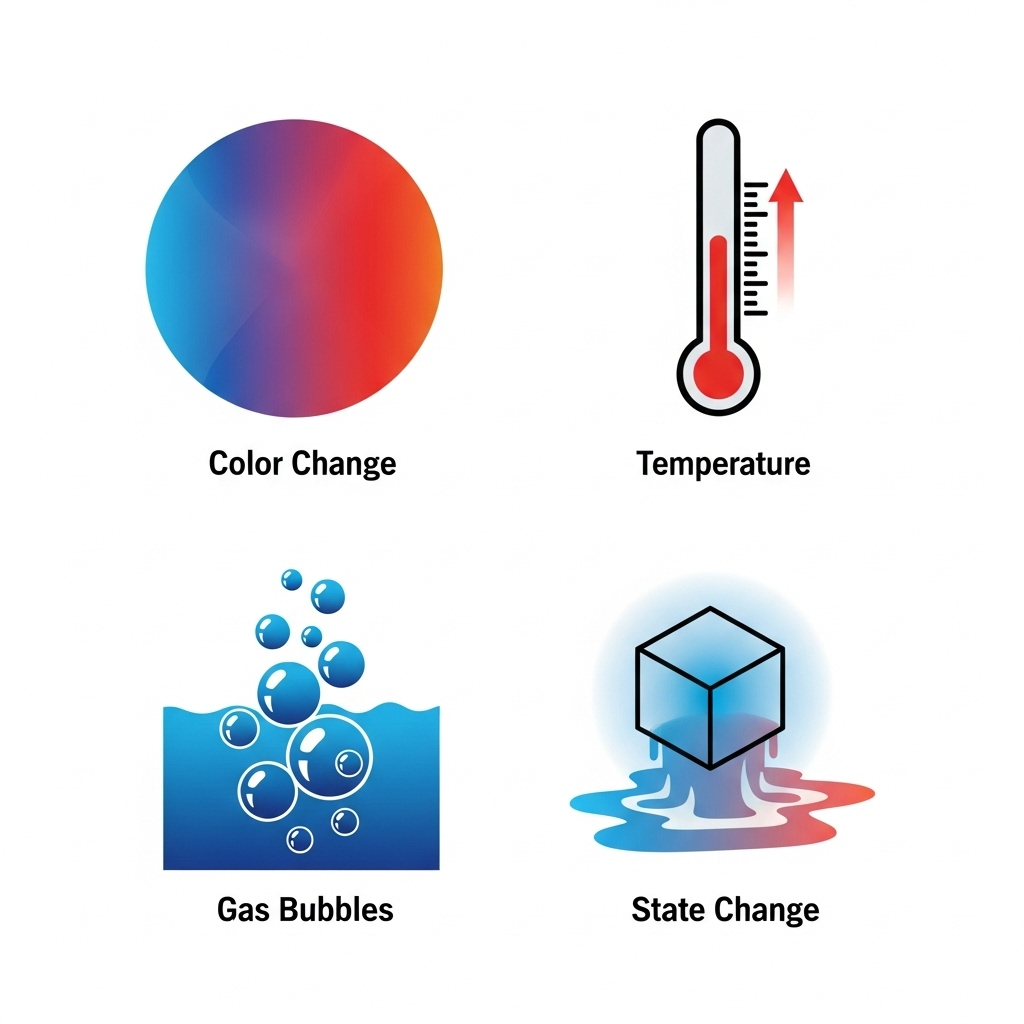
- Observations indicating a chemical reaction: We can determine if a chemical reaction has taken place by observing:
- Change in state
- Change in color
- Evolution of a gas
- Change in temperature
- Examples from daily life:
- Milk left at room temperature during summers
- An iron tawa/pan/nail left exposed to humid atmosphere (rusting)
- Grapes getting fermented
- Food being cooked
- Food getting digested in our body
- Respiration
1.1 Chemical Equations
- Word-Equation: A chemical reaction can be described in a shorter form using a word-equation.
- Format: Reactants are written on the left-hand side (LHS) with a plus sign (+) between them. Products are written on the right-hand side (RHS) with a plus sign (+) between them. An arrow placed between them shows the change of reactants to products and indicates the direction of the reaction.
- Reactants: Substances that undergo chemical change.
- Products: New substances formed during the reaction.
- Example (Activity 1.1): Magnesium + Oxygen → Magnesium oxide.
1.1.1 Writing a Chemical Equation
- Using Chemical Formulae: Chemical equations can be made more concise and useful by using chemical formulae instead of words. A chemical equation represents a chemical reaction.
- Example: Mg+O2→MgO.
- Skeletal Chemical Equation: If the number of atoms of each element is not the same on both sides of the equation, it is unbalanced because the mass is not the same on both sides. Such an equation is called a skeletal chemical equation. For example, Equation (1.2) (Mg+O2→MgO) is a skeletal chemical equation for the burning of magnesium in air.
1.1.2 Balanced Chemical Equations
- Law of Conservation of Mass: Mass can neither be created nor destroyed in a chemical reaction. This means the total mass of the elements present in the products must be equal to the total mass of the elements present in the reactants. In other words, the number of atoms of each element remains the same before and after a chemical reaction.
- Necessity of Balancing: Chemical equations need to be balanced to satisfy the law of conservation of mass.
- Hit-and-Trial Method for Balancing:
- Step I: Draw boxes around each formula; do not change anything inside the boxes while balancing.
- Example: [Fe]+[H2O]→[Fe3O4]+[H2].
- Step II: List the number of atoms of different elements in the unbalanced equation.
- Step III: Start balancing with the compound having the maximum number of atoms. Select the element within that compound which has the maximum number of atoms.
- Example for Fe+H2O→Fe3O4+H2: Select Fe3O4 and oxygen. To balance oxygen, put coefficient ‘4’ as 4H2O. The partly balanced equation becomes Fe+4H2O→Fe3O4+H2.
- Step IV: Balance other elements (e.g., hydrogen). To equalize H atoms, make the number of molecules of hydrogen as four on the RHS.
- The equation becomes Fe+4H2O→Fe3O4+4H2.
- Step V: Balance the remaining element (e.g., iron). To equalize Fe, take three atoms of Fe on the LHS.
- The equation becomes 3Fe+4H2O→Fe3O4+4H2.
- Step VI: Check the correctness by counting atoms of each element on both sides. If equal, the equation is balanced.
- Example: 3Fe+4H2O→Fe3O4+4H2.
- Step I: Draw boxes around each formula; do not change anything inside the boxes while balancing.
- Writing Symbols of Physical States: To make a chemical equation more informative, physical states are mentioned along with chemical formulae.
- (g) for gaseous, (l) for liquid, (aq) for aqueous (solution in water), and (s) for solid.
- Example: 3Fe(s)+4H2O(g)→Fe3O4(s)+4H2(g).
- Physical states are usually not included unless necessary.
- Reaction Conditions: Temperature, pressure, catalyst, etc., can be indicated above or below the arrow.
- Example: CO(g)+2H2(g)340 atm
CH3OH(l).
- Example: 6CO2(aq)+12H2O(l)Sunlight
ChlorophyllC6H12O6(aq)+6O2(aq)+6H2O(l).
- Example: CO(g)+2H2(g)340 atm
1.2 Types of Chemical Reactions
1.2.1 Combination Reaction
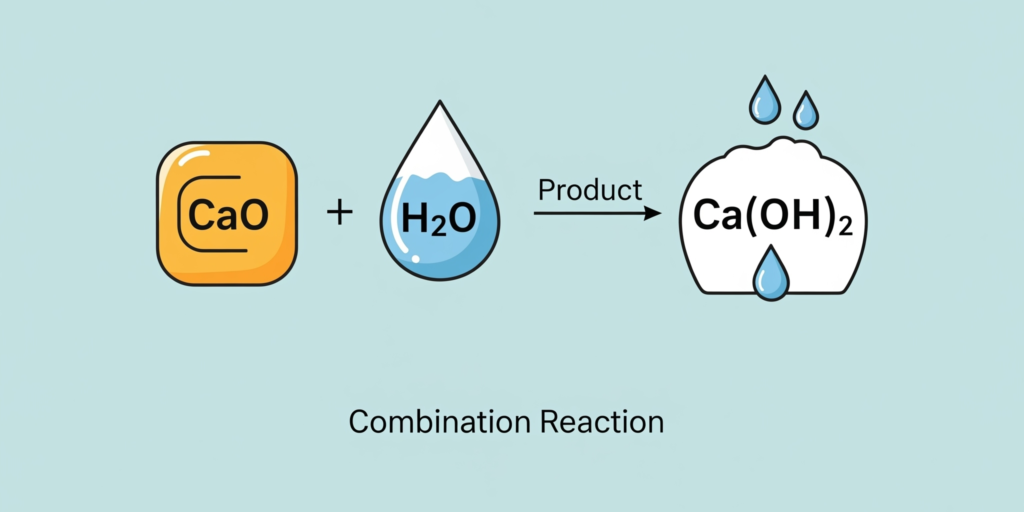
- Definition: A reaction in which a single product is formed from two or more reactants.
- Example (Activity 1.4): Calcium oxide (quick lime) reacts vigorously with water to produce slaked lime (calcium hydroxide).
- CaO(s)+H2O(l)→Ca(OH)2(aq)+Heat.
- Other Examples:
- Burning of coal: C(s)+O2(g)→CO2(g).
- Formation of water: 2H2(g)+O2(g)→2H2O(l).
- Exothermic Reactions: Reactions in which heat is released along with the formation of products. The reaction mixture becomes warm. Combination reactions often release heat.
- Examples:
- Burning of natural gas: CH4(g)+2O2(g)→CO2(g)+2H2O(g).
- Respiration: C6H12O6(aq)+6O2(aq)→6CO2(aq)+6H2O(l)+energy.
- Decomposition of vegetable matter into compost.
- Examples:
1.2.2 Decomposition Reaction
- Definition: A single reactant breaks down to give simpler products. These reactions require energy in the form of heat, light, or electricity for breaking down the reactants.
- Thermal Decomposition: When a decomposition reaction is carried out by heating, it is called thermal decomposition.
- Example (Activity 1.5): Decomposition of ferrous sulphate crystals. Green ferrous sulphate crystals (FeSO4⋅7H2O) lose water and decompose to ferric oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide (SO3) upon heating.
- 2FeSO4(s)Heat
Fe2O3(s)+SO2(g)+SO3(g).
- 2FeSO4(s)Heat
- Example: Decomposition of calcium carbonate (limestone) to calcium oxide (quick lime) and carbon dioxide on heating.
- CaCO3(s)Heat
CaO(s)+CO2(g).
- CaCO3(s)Heat
- Example (Activity 1.6): Heating lead nitrate powder, producing brown fumes of nitrogen dioxide (NO2).
- 2Pb(NO3)2(s)Heat
2PbO(s)+4NO2(g)+O2(g).
- 2Pb(NO3)2(s)Heat
- Example (Activity 1.5): Decomposition of ferrous sulphate crystals. Green ferrous sulphate crystals (FeSO4⋅7H2O) lose water and decompose to ferric oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide (SO3) upon heating.
- Electrolytic Decomposition (Activity 1.7 – Electrolysis of water): Decomposition by electricity.
- H2O is decomposed into hydrogen gas (H2) and oxygen gas (O2). The volume of hydrogen gas collected is double the amount of oxygen gas collected.
- Photolytic Decomposition (Activity 1.8): Decomposition by light.
- Example: White silver chloride turns grey in sunlight due to decomposition into silver and chlorine.
- 2AgCl(s)Sunlight
2Ag(s)+Cl2(g).
- 2AgCl(s)Sunlight
- Example: Silver bromide also decomposes in the same way.
- 2AgBr(s)Sunlight
2Ag(s)+Br2(g).
- 2AgBr(s)Sunlight
- These reactions are used in black and white photography.
- Example: White silver chloride turns grey in sunlight due to decomposition into silver and chlorine.
- Endothermic Reactions: Reactions in which energy is absorbed. Decomposition reactions are generally endothermic.
- Example: Reaction of barium hydroxide with ammonium chloride, where the bottom of the test tube feels cold.
1.2.3 Displacement Reaction
- Definition: A reaction where one element displaces or removes another element from its compound.
- Activity 1.9 (Iron nails in copper sulphate solution): Iron nails become brownish, and the blue color of copper sulphate solution fades.
- Fe(s)+CuSO4(aq)→FeSO4(aq)+Cu(s).
- Iron displaces copper from copper sulphate solution.
- Other Examples:
- Zn(s)+CuSO4(aq)→ZnSO4(aq)+Cu(s).
- Pb(s)+CuCl2(aq)→PbCl2(aq)+Cu(s).
- Zinc and lead are more reactive than copper and displace copper from its compounds.
1.2.4 Double Displacement Reaction
- Definition: Reactions in which there is an exchange of ions between the reactants. Two different atoms or groups of atoms (ions) are exchanged.
- Activity 1.10: Mixing sodium sulphate solution and barium chloride solution.
- Observation: A white substance insoluble in water is formed.
- This insoluble substance is called a precipitate.
- The reaction is: Na2SO4(aq)+BaCl2(aq)→BaSO4(s)+2NaCl(aq).
- The white precipitate of BaSO4 is formed by the reaction of SO42− and Ba2+.
- Precipitation Reaction: Any reaction that produces a precipitate can be called a precipitation reaction. Precipitation reactions produce insoluble salts.
1.2.5 Oxidation and Reduction (Redox Reactions)
- Oxidation: If a substance gains oxygen or loses hydrogen during a reaction, it is said to be oxidized.
- Reduction: If a substance loses oxygen or gains hydrogen during a reaction, it is said to be reduced.
- Redox Reactions (Oxidation-Reduction Reactions): Reactions where one reactant gets oxidized while the other gets reduced.
- Activity 1.11 (Heating copper powder):
- Copper powder’s surface becomes coated with black copper(II) oxide because oxygen is added to copper.
- 2Cu+O2Heat
2CuO. (Copper is oxidized)
- 2Cu+O2Heat
- If hydrogen gas is passed over heated copper(II) oxide, the black coating turns brown as copper is obtained.
- CuO+H2Heat
Cu+H2O.
- In this reaction, copper(II) oxide is losing oxygen (reduced), and hydrogen is gaining oxygen (oxidized).
- CuO+H2Heat
- Copper powder’s surface becomes coated with black copper(II) oxide because oxygen is added to copper.
- Other Examples of Redox Reactions:
- ZnO+C→Zn+CO. (Carbon is oxidized to CO, ZnO is reduced to Zn ).
- MnO2+4HCl→MnCl2+2H2O+Cl2. (HCl is oxidized to Cl2, MnO2 is reduced to MnCl2 ).
1.3 Effects of Oxidation Reactions in Everyday Life
1.3.1 Corrosion
- Definition: When a metal is attacked by substances around it, such as moisture, acids, etc., it is said to corrode. This process is called corrosion.
- Examples:
- Rusting of iron (reddish-brown powder coating on iron articles).
- Black coating on silver.
- Green coating on copper.
- Impact: Corrosion causes damage to car bodies, bridges, iron railings, ships, and all objects made of metals, especially iron. It is a serious problem, requiring significant expenditure for replacement of damaged iron.
1.3.2 Rancidity
- Definition: When fats and oils (in food materials) are oxidized, they become rancid, and their smell and taste change.
- Prevention:
- Adding antioxidants (substances preventing oxidation) to foods containing fats and oil.
- Keeping food in airtight containers to slow down oxidation.
- Flushing bags of chips with gases like nitrogen to prevent oxidation.